In vivo CRISPR screen reveals regulation of macrophage states in neuroinflammation
Neuroimmunological diseases are autoimmune diseases of the nervous system. In autoimmune diseases, the immune system "mistakenly" targets the body's own structures. By far the most common neuroimmunological disease is multiple sclerosis.
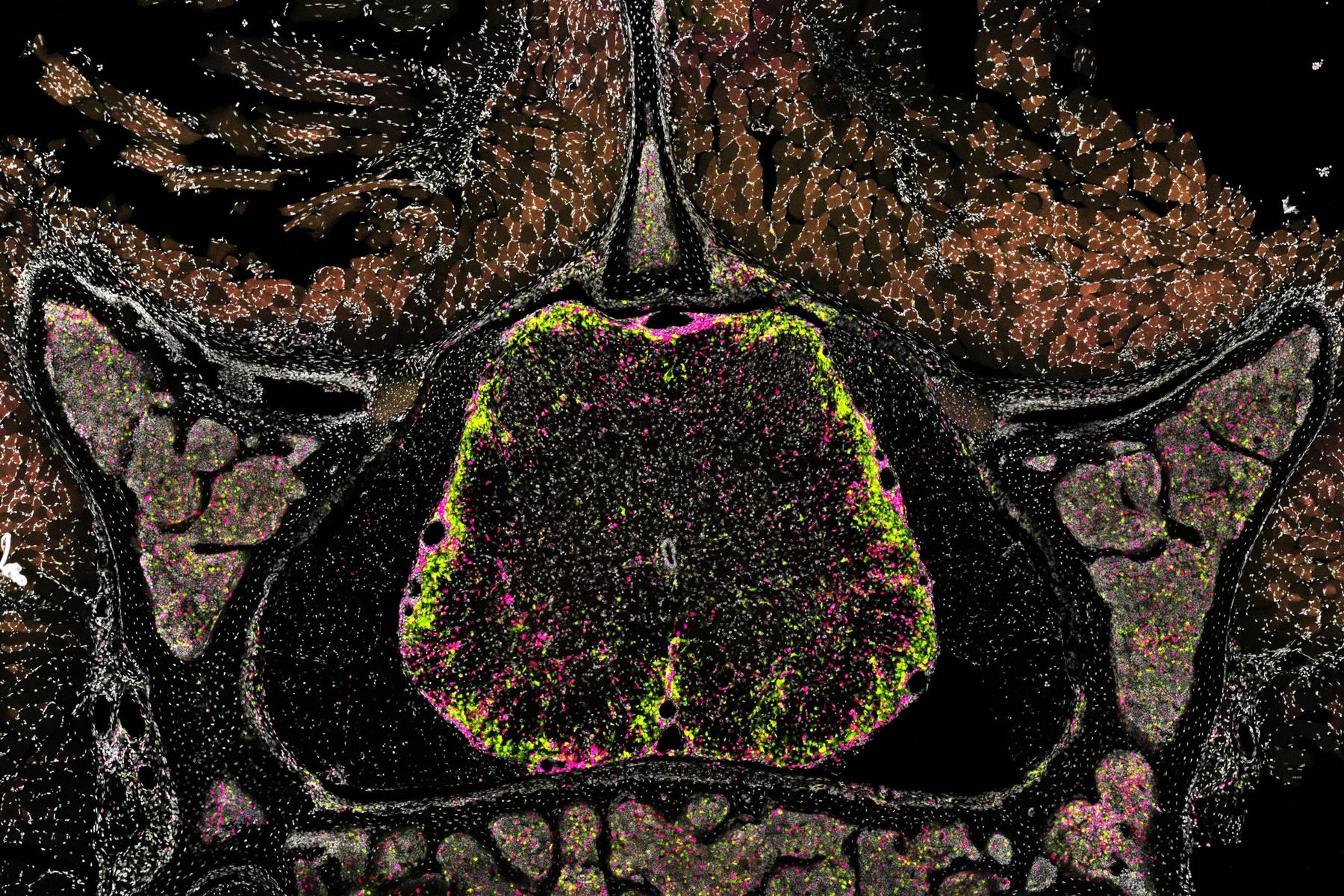
Using an in vivo CRISPR screening system, the group has mapped the signals that shape macrophage activity in mouse models of multiple sclerosis (MS), providing new insight into the cellular mechanisms that drive disease progression. The team’s method focuses on genetically editable progenitor cells, which can be introduced into the bloodstream and tracked as they develop into the full range of blood-derived myeloid cells within the central nervous system. This approach allowed the researchers to carry out a large-scale screen of more than 100 cytokine receptors and signalling molecules directly in living animals.
Several pathways emerged as particularly influential. Interferon-γ, tumour necrosis factor-α, granulocyte-macrophage colony-stimulating factor and transforming growth factor-β were identified as key regulators of macrophage polarisation during neuroinflammation. These findings were supported by single-cell transcriptomic analyses, which confirmed that the transferred progenitor cells reliably generated all major myeloid cell types found in the inflamed CNS.
By integrating this system with Perturb-seq and biosensor readouts in vivo, the researchers around Martin Kerschensteiner were able to observe how cytokine signals affect not only gene expression but also cell behaviour, including migration, debris clearance and oxidative activity. Comparative analyses further showed that many of these cytokine-driven signatures are conserved across different myeloid populations, tissue compartments and even species. This alignment provides a valuable point of reference for understanding related processes in the cerebrospinal fluid and brain tissue of people with MS.
The resulting pipeline is both rapid and scalable, offering a practical route to studying myeloid cell states with high resolution. More broadly, it highlights the cytokine cues that control these cells in MS and its experimental models.
Taken together, the work represents a significant step towards a more precise understanding of innate immune regulation in neuroinflammatory disease. By identifying the molecular signals that shape myeloid behaviour, the study may ultimately support the development of therapeutic strategies designed to modulate these cells more selectively in MS.
Publication: Clara de la Rosa and Arek Kendirli et al.: In vivo CRISPR screen reveals regulation of macrophage states in neuroinflammation. Nature Neuroscience, December 2025